We continue to develop a range of chemistry-led approaches to interrogate novel targets/techniques for the treatment of disease. Our studies span efforts in curiosity-led basic science through to translational drug discovery. These include medicinal chemistry efforts towards a wide range of current and future drug targets and the development of chemical biological tools that would enable better biological understanding of disease-related events. We have a strong interest in the use of photochemically-active reagents in chemical biology, particularly in the context of photopharmacology.
Chemical Biology, Medicinal Chemistry and Drug Discovery
Therapeutics targeting transcriptional pathways
Tight control of gene transcription is essential for cellular identity and dysregulation of transcriptional programmes is a common mechanism that leads to the development and maintenance of disease. Thus, molecular mediators of transcriptional processes represent important and exciting targets for new therapies and define many of the current translational projects in the group. Representative highlights of our recent work include:
Cyclin-dependent kinase 7 (CDK7). CDK7 is an enzyme which phosphorylates RNA polymerase II (Pol II) at active gene promoters in order to permit transcription. Frequent misregulation of transcription via aberrant CDK7 levels or signaling has suggested CDK7 as a cancer target. We were previously involved in a large drug discovery team effort at Imperial College that took this project from a clinical hypothesis and virtual chemical scaffolds to a clinical drug candidate ICEC0942. This molecule, now called Samuraciclib, was licenced to Carrick Therapeutics and is currently in Phase II clinical trials for cancer with FDA fast track status for breast cancer. Representative publications: Mol. Cancer Ther. 2018, 17, 1156. DOI; ChemMedChem 2017, 12, 372. DOI; Cancer Res. 2009, 69, 6208. DOI.
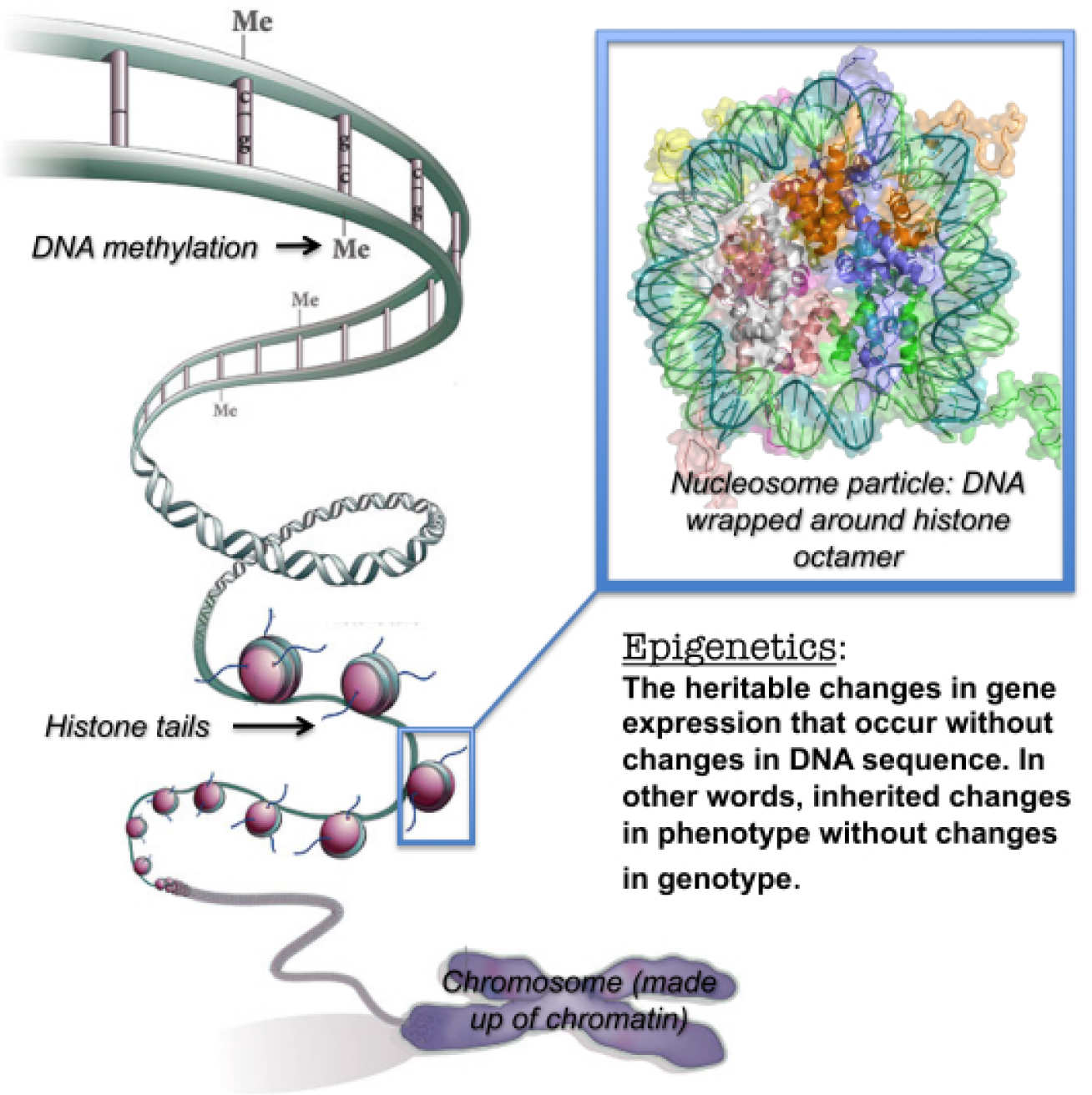
Epigenetic targets. The human genome is packaged into chromatin, a macromolecular complex consisting of DNA, histone and non-histone proteins. Chromatin structure and signalling play a critically important role in transcriptional regulation via so-called epigenetic processes. Histone post-translational modifications (including acetylation, methylation, phosphorylation, etc.) are key mediators in epigenetic transcriptional control; thus the proteins responsible for the regulation of these epigenetic ‘marks’ represent interesting drug targets. Representative ongoing projects include the development of inhibitors against histone deacetylase and histone lysine methyltransferase enzymes:
The histone lysine methyltransferases (HKMT) as drug targets.
(i) Cancer. HKMTs are enzymes that transfer one or more methyl groups to specific lysine residues on histone (and non-histone) proteins. Many cancers show dependencies on the histone methyltransferases to maintain an aberrant epigenetic state. Together with Professor Bob Brown, we have found that dual pharmacological inhibition of the repressive HKMTs EHMT2 and EZH2 yields greater activity than selective inhibition of either enzyme. We have identified dual EHMT2/EZH2 inhibitors and continue to explore the application of these proof-of-concept compounds in a range of cancer subtypes/therapeutic combinations, including as an immuno-oncology approach to ovarian cancer. Representative publications: Front. Cell Dev. Biol. 2023, 11, 1076458. DOI; Mol. Cancer. Ther. 2022, 21, 522. DOI; Clin. Epigenetics 2015, 7, 84 DOI.
(ii) Malaria. Together with Professor Artur Scherf we were the first to discover Plasmodium histone methyltransferase (PfHKMT) inhibitors that result in blood stage independent parasite killing. In collaboration with Prof. Dominique Mazier we also discovered our inhibitors to have the unprecedented ability to ‘reawaken’ dormant liver stage parasites. Dormant and largely drug resistant Plasmodium parasites (in P. vivax) cause recurrent malaria and are a significant and largely untreatable clinical problem; this drug induced ‘awakening’ approach holds much hope to tackle such a limitation and therefore provide a new therapeutic option for this disease. Representative publications: MedChemComm 2017, 8, 1069. DOI; Nature Med. 2014, 20, 307. DOI; P. Natl. Acad. Sci. USA 2012, 109, 16708. DOI.
Nuclear receptors. The nuclear receptor REV-ERB is a transcriptional regulator involved in the regulation of many physiological processes, from circadian rhythm, to immune function and metabolism. Accordingly, REV-ERB has been considered a promising, but difficult drug target for the treatment of numerous diseases. In collaboration with Professor Hugh Brady, we have been exploring the potential for REV-ERB inhibition in a cell expansion process for the production of natural killer (NK) cells. NK cells are critical immune effector cells and the adoptive transfer of large numbers of cytolytic NK cells represents a rapidly developing cancer immunotherapeutic approach. Our proprietary data has demonstrated a promising and exciting approach to the generation of very large numbers of highly active NK cells for use as an adoptive cell therapy. This research led to the formation of NK:IO Ltd. Representative publications: Bioorg. Med. Chem. Lett. 2020, 30, 127395. DOI.
Other therapeutics projects
Outside of our core interests in the development of therapeutic approaches towards transcriptional targets, we are working on a range of other collaborative and translational projects, which leverage the strong biological research at Imperial and its partner institutes (Crick, MRC LMS, etc.). These projects span a range of target classes – kinases, DNA processing and repair proteins, motor proteins – and a range of diseases – cancer, infectious diseases (e.g. malaria,), cardiovascular diseases, neurological diseases, etc.
 As a representative example, in collaboration with Professor Jake Baum, we have been pursuing unique transmission-blocking anti-malarial compounds. In malaria management, there is an increasing interest in the discovery of novel drugs that target parasite passage from the infected individual to the feeding mosquito and back again. The action of such therapeutics is geared at the population level and could result in the eventual eradication of malaria. Following an in-house high throughput screen, DDD881 was identified as an exciting new hit compound that appeared to block parasite transmission by specifically targeting the transformation of the sexual stages of Plasmodium progression. We have conducted further hit to lead medicinal chemistry on DDD881 and identified Plasmodium falciparum 16 kDa parasitophorous vacuole membrane (PVM) protein Pfs16 as the target of this compound. Representative publications: Dis. Model Mech. 2023, 16, dmm049950. DOI; J. Med. Chem. 2020, 63, 2240. DOI; Curr. Opin. Chem. Biol. 2019, 50, 1. DOI; Nature Commun. 2018, 9, 3805. DOI
As a representative example, in collaboration with Professor Jake Baum, we have been pursuing unique transmission-blocking anti-malarial compounds. In malaria management, there is an increasing interest in the discovery of novel drugs that target parasite passage from the infected individual to the feeding mosquito and back again. The action of such therapeutics is geared at the population level and could result in the eventual eradication of malaria. Following an in-house high throughput screen, DDD881 was identified as an exciting new hit compound that appeared to block parasite transmission by specifically targeting the transformation of the sexual stages of Plasmodium progression. We have conducted further hit to lead medicinal chemistry on DDD881 and identified Plasmodium falciparum 16 kDa parasitophorous vacuole membrane (PVM) protein Pfs16 as the target of this compound. Representative publications: Dis. Model Mech. 2023, 16, dmm049950. DOI; J. Med. Chem. 2020, 63, 2240. DOI; Curr. Opin. Chem. Biol. 2019, 50, 1. DOI; Nature Commun. 2018, 9, 3805. DOI
Drug discovery science beyond medicinal chemistry
We continue to seek interesting ways to couple our medicinal chemistry endeavours to broader therapeutic approaches. For example, in collaboration with scientists at CSIRO, we have been developing polymeric delivery systems that link a delivery hypothesis to the medicinal chemistry of a particular target of interest in cancer. Representative publication: ACS Appl. Bio Mater. 2020, 3, 5775. DOI; Polym. Chem. 2018, 9, 131. DOI.

 Where required, we apply chemical biological target validation approaches to inhibitors developed in our medicinal chemistry efforts. We have particular expertise in photochemical techniques and, as such, several of these studies focus on the use of photoaffinity labelling. For example, we have previously developed a small-molecule photo-cross-linkable probe to investigate the targets of our diaminoquinazoline series of PfHKMT inhibitors. We have demonstrated the effectiveness of our designed probe for photoaffinity labelling of Plasmodium lysates and continue to use these tools in ongoing target validation studies. In collaboration with Prof. Ed Tate, we have also contributed to the characterisation of photochemical tools to identify the small molecule binding site in hedgehog acyltransferase (HHAT). Representative publications: Angew. Chem. Int. Ed. 2021, 60, 13542. DOI; ACS Infect. Dis. 2018, 4, 523. DOI.
Where required, we apply chemical biological target validation approaches to inhibitors developed in our medicinal chemistry efforts. We have particular expertise in photochemical techniques and, as such, several of these studies focus on the use of photoaffinity labelling. For example, we have previously developed a small-molecule photo-cross-linkable probe to investigate the targets of our diaminoquinazoline series of PfHKMT inhibitors. We have demonstrated the effectiveness of our designed probe for photoaffinity labelling of Plasmodium lysates and continue to use these tools in ongoing target validation studies. In collaboration with Prof. Ed Tate, we have also contributed to the characterisation of photochemical tools to identify the small molecule binding site in hedgehog acyltransferase (HHAT). Representative publications: Angew. Chem. Int. Ed. 2021, 60, 13542. DOI; ACS Infect. Dis. 2018, 4, 523. DOI.
Photopharmacology is a rapidly growing approach that uses light to change the shape and/or properties of a therapeutic agent. Such photoswitching, in turn, changes the biological activity of the compound. Therefore, in its most simple form, photopharmacology is an approach to switch the activity of a drug ‘on’ and ‘off’ using light. Coupling our work on novel photoswitchable molecules to our interests in medicinal chemistry and chemical biology, we have a large number of ongoing collaborative projects aimed at the generation of photopharmacological agents. To date, we have published examples of heteroaromatic photoswitchable aminohydrolyse inhibitors as a photopharmacological antimicrobial approach, the ability to control targeted protein degradation with light using heteroaromatic azo PROTACs and the ability to control electrically excitable cells using a photoswitchable TRPA1 ligand that we called TRPswitch.
 This latter study was particularly exciting as it allowed us to demonstrate photocontrol of physiology in live zebrafish larvae, using our heteroaromatic switches. The TRPA1/TRPswitch combination has a number of key advantages over other chemo-optogenetic approaches and should be particularly applicable in systems where a large depolarization current is needed or sustained channel activation is desirable. Representative publications: Chem. Commun. 2022, 58, 10933. DOI; J. Am. Chem. Soc. 2020, 142, 17457. DOI; J. Med. Chem. 2020, 63, 11436. DOI; ACS Infect. Dis. 2017, 3, 152. DOI.
This latter study was particularly exciting as it allowed us to demonstrate photocontrol of physiology in live zebrafish larvae, using our heteroaromatic switches. The TRPA1/TRPswitch combination has a number of key advantages over other chemo-optogenetic approaches and should be particularly applicable in systems where a large depolarization current is needed or sustained channel activation is desirable. Representative publications: Chem. Commun. 2022, 58, 10933. DOI; J. Am. Chem. Soc. 2020, 142, 17457. DOI; J. Med. Chem. 2020, 63, 11436. DOI; ACS Infect. Dis. 2017, 3, 152. DOI.
Contact
Matthew J. Fuchter FRSC
Professor of Chemistry
Department of Chemistry
Molecular Sciences Research Hub, White City Campus
Wood Lane, London, W12 OBZ
m.fuchter@imperial.ac.uk
Tel: +44 (0)20 8594 5815